COVID-19 Protocol
Overview
The COVID-19 Protocol is a new procedure for determining the feasibility and ethics of carrying out research during the COVID-19 pandemic.
This protocol applies to projects supported by the EGAP network during the COVID-19 pandemic (including the projects in the Metaketa V round and research associated with the COVID-19 Small Grants fund and Priority Theme Small Grants fund).
Principal Investigators who wish to carry out research are required to complete a set of materials describing the steps and precautions taken with regard to the pandemic. These forms will then be reviewed by EGAP to decide whether the research meets the established precautionary standards.
Please see the complete process for initiation / resumption of research activities during the COVID-19 pandemic along with the application forms below.
Note: These documents were developed using resources available from the University of Chicago; Toronto Metropolitan University; the University of California, Berkeley; Innovations for Poverty Action; J-PAL; the World Bank; and others.
All Principal Investigators seeking to begin or continue research activities funded by EGAP during the COVID-19 pandemic are required to follow the below process for gaining research activity approval. This includes fully remote activities (e.g., online surveys, phone surveys) associated with research as well as in-person field activities. In-person field research activities include activities associated with either research interventions or data collection, including, but not limited to, in-person meetings, face-to-face surveys, group discussions, or other activities that would have two or more individuals from distinct households interacting in person.
Research Activities that Involve Field-Related, Travel-Related, or In-Person Activities
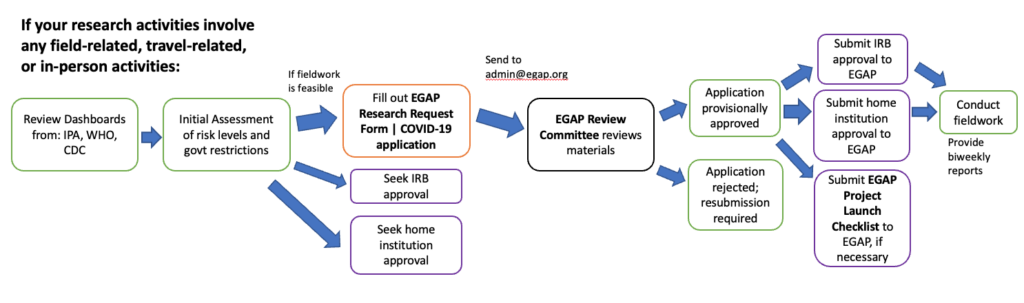
If your research activities involve any field-related, travel-related, or in-person activities, EGAP requires the following review process:
- Conduct an initial assessment by reviewing the IPA Dashboard, the CDC Dashboard, and the WHO Dashboard to learn about risk levels and country government restrictions. Field research activities would be permitted only if a compelling case could be made that they would not contravene local restrictions and that they would not introduce risks beyond those that prevail for activities that are commonly undertaken in the population in question.
- If a case can be made for in-person research after the initial assessment, then fill out the Research Request Form | COVID-19.
- Submit the application to EGAP at admin@egap.org.
- EGAP will review the form. Possible outcomes include:
- Conditional approval, with the requirement of steps 5-7 below. Steps 8 and 9 may or may not be required, depending on the risk level of the project country.
- Application is rejected, resubmission is required to move forward with research.
- Seek home institution approval, as per the requirements of all principal investigators’ home institutions, where applicable.
- Seek IRB approval for initiation or resumption of field research from all principal investigators’ home institutions, where applicable.
- Submit home institution and IRB approvals to EGAP.
- Fill out the In-Person Data Collection Project Launch Checklist, if requested by EGAP. (Note that both the application and checklist may be useful for home institution permissions and IRB applications.)
- Provide monthly email updates about research activities that includes any changes to COVID-related issues in the study country and globally; confirms that the Project Launch Checklist is being respected; and explains any other issues that have arisen.
Research Activities that DO NOT Involve Field-Related, Travel-Related, or In-Person Activities
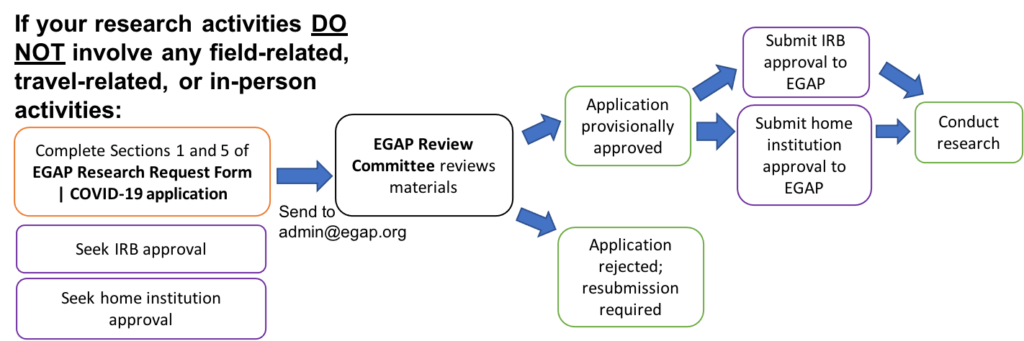
If your research activities DO NOT involve any field-related, travel-related, or in-person activities, EGAP requires the following review process to confirm that there are no COVID-related risks associated with your research:
- Fill out sections 1 and 5 of the Research Request Form | COVID-19.
- Submit the application to EGAP at admin@egap.org. (Note that the application may be useful for home institution permissions and IRB applications.)
- EGAP Review Committee will review materials. Possible outcomes include:
- Conditional approval, with the requirement of steps 4-6 below.
- Application is rejected.
- Seek home institution approval, as per the requirements of all principal investigators’ home institutions, where applicable.
- Seek IRB approval for initiation or resumption of field research from all principal investigators’ home institutions, where applicable.
- Submit home institution and IRB approvals to EGAP.
NOTE: Home institution approval and IRB approval requests can be submitted simultaneously with your EGAP approval request.
Please find below the required application form and project launch checklist.